
Electronegativity
How strongly an element holds on to it’s bond
The term “electronegativity” was introduced in 1811 by Swedish Chemist Baron Jons Jacob Berzelius (one of the founders of modern chemistry with the holiday Berzelius Day celebrated on August 20th)
Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. It depends on several factors, including its nuclear charge, the distance between the nucleus and the valence electrons, and the shielding effect of inner electrons
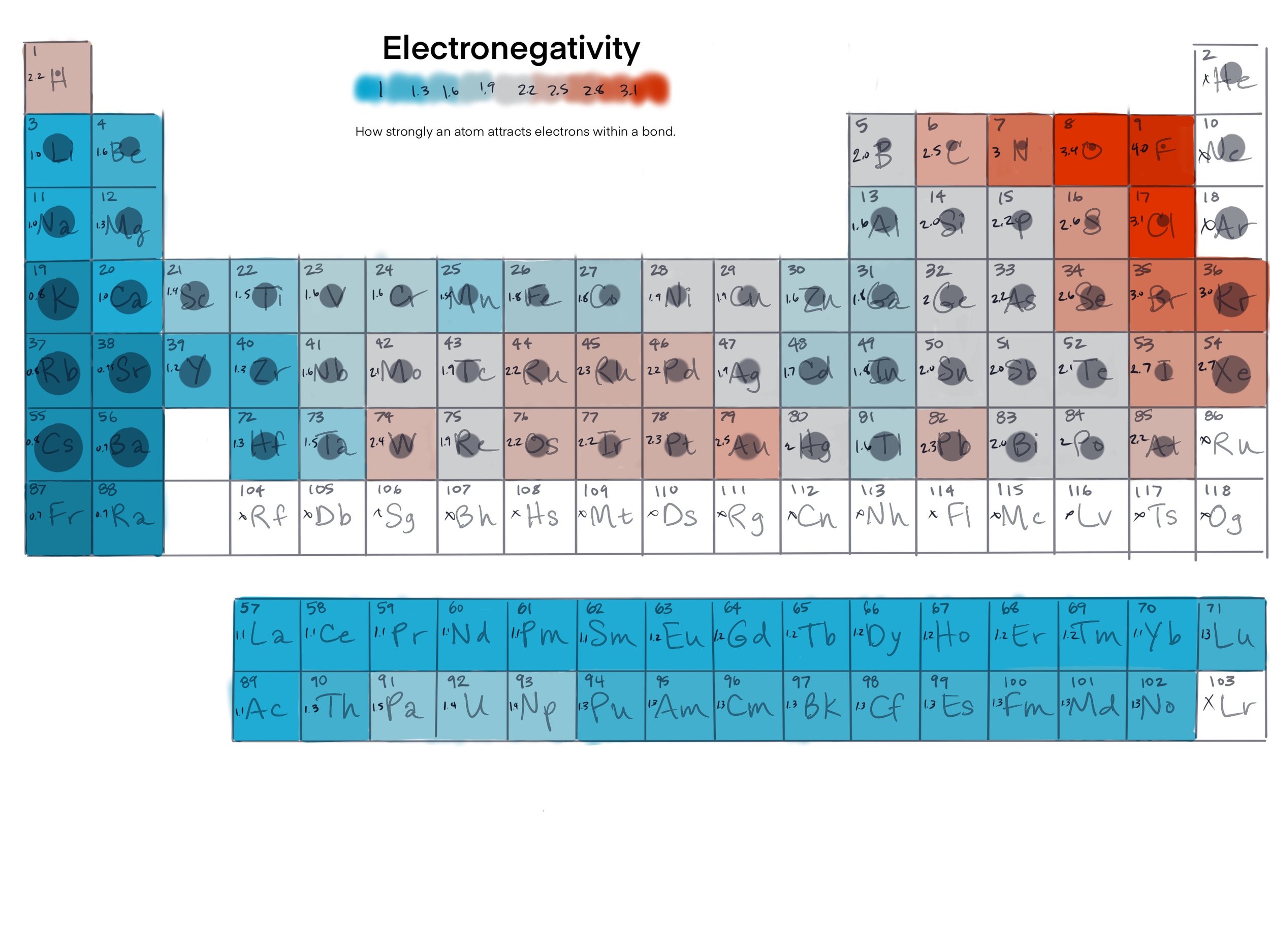
An accurate scale of electronegativity was not developed until 1932 when American Chemist and two-time Nobel Prize winner, Linus Pauling, proposed a scale based on each element’s ability to attract electrons in a bond. His scale, known as the Pauling electronegativity scale, assigns values ranging from 0.7 to 4.0 to each element, with higher values indicating a greater electronegativity. The periodic table below highlights the electronegativity of the elements with high electronegativities in red (they strongly attract electrons).
Types of Bonds based on Electronegativity
-
When the difference in electronegativity between two atoms is very small, typically less than 0.5, the bond is considered to be a nonpolar covalent bond. In this type of bond, the electrons are shared equally between the two atoms, and there is no separation of charge. Examples of nonpolar covalent bonds include the bond between two hydrogen atoms in H2 and the bond between two chlorine atoms in Cl2.
-
When the difference in electronegativity between two atoms is between 0.5 and 1.7, the bond is considered to be a polar covalent bond. In this type of bond, one atom has a slightly greater electronegativity than the other, resulting in a partial separation of charge. Examples of polar covalent bonds include the bond between hydrogen and oxygen in water (H2O) and the bond between carbon and oxygen in carbon dioxide (CO2).
-
When the difference in electronegativity between two atoms is greater than 1.7, the bond is considered to be an ionic bond. In this type of bond, one atom has a much greater electronegativity than the other, resulting in the complete transfer of electrons from one atom to the other. The resulting ions have opposite charges and are held together by electrostatic attractions. Examples of ionic bonds include the bond between sodium and chlorine in sodium chloride (NaCl) and the bond between magnesium and oxygen in magnesium oxide (MgO).
Examples
-
In the case of a Si-O bond in a silicate tetrahedron, you can use the Pauling electronegativity values for silicon and oxygen to calculate the electronegativity difference:
Electronegativity of oxygen (O) = 3.44
Electronegativity of silicon (Si) = 1.90
Electronegativity difference (ΔEN) = |3.44 - 1.90| = 1.54
The electronegativity difference between oxygen and silicon in a Si-O bond is 1.54. This value suggests that the bond between Si and O has a polar covalent character, as the electrons are shared unequally between the two atoms, with oxygen attracting the electrons more strongly than silicon.
-
The electronegativity difference between oxygen and hydrogen in an O-H bond within a water molecule can be calculated using the Pauling electronegativity scale:
Electronegativity of oxygen (O) = 3.44
Electronegativity of hydrogen (H) = 2.20
Electronegativity difference (ΔEN) = |3.44 - 2.20| = 1.24
The electronegativity difference between oxygen and hydrogen in an O-H bond is 1.24. This value indicates that the O-H bond in a water molecule has a polar covalent character, with the oxygen atom attracting the bonding electrons more strongly than the hydrogen atom. This unequal sharing of electrons results in a polar bond, contributing to the overall polarity of the water molecule.
