Orbitals
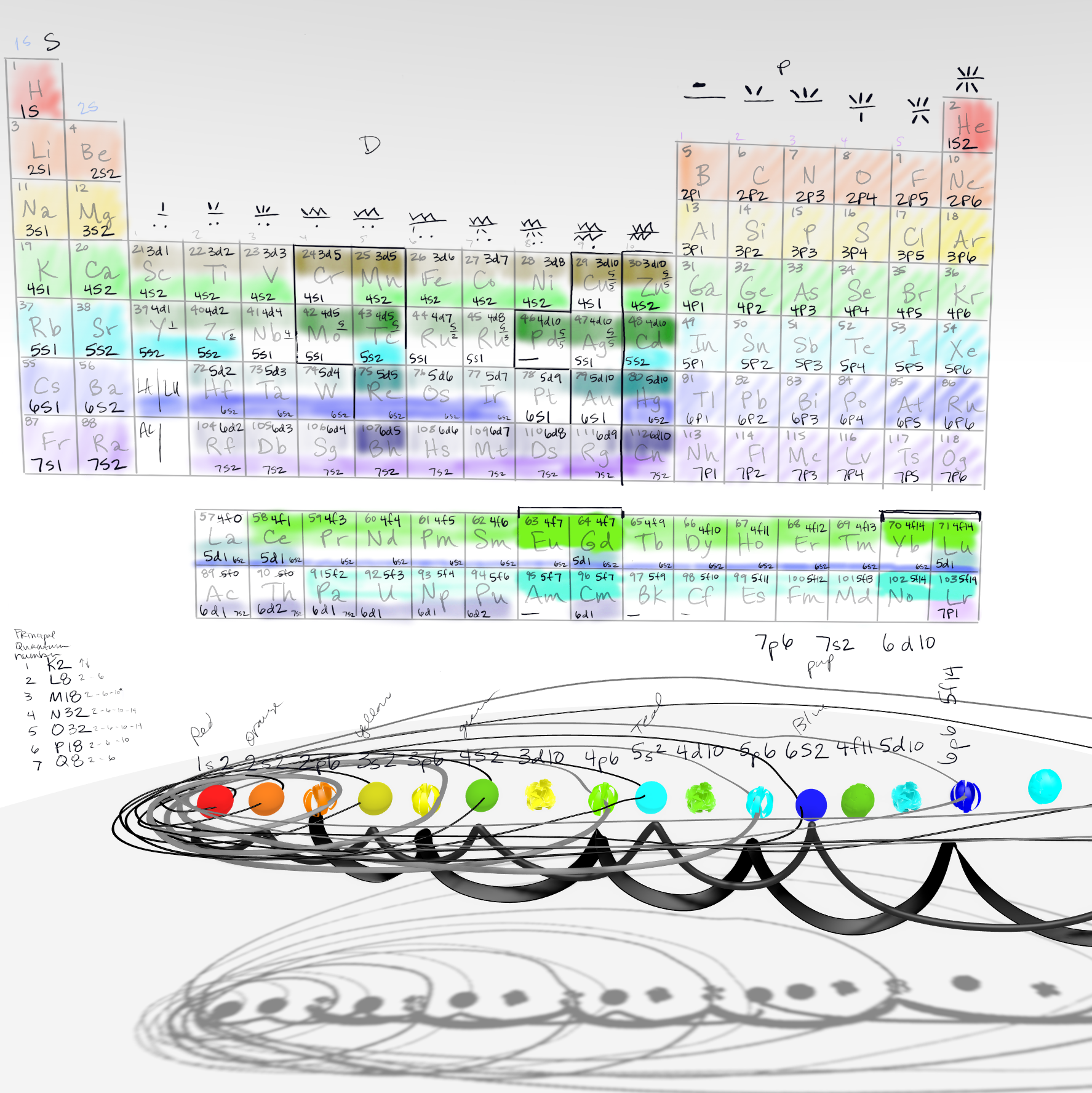
Principal Quantum Numbers and Atomic Subshells
Principal Quantum Number
The principal quantum number is a term used in quantum mechanics to describe the energy levels of an atom's electrons. It is denoted by the letter "n," and it can take on integer values ranging from 1 to infinity.
The principal quantum number determines the size and energy of an electron's orbital, or the region of space where the electron is most likely to be found. Specifically, as the value of n increases, the size and energy of the orbital increase as well. For example, an electron with a principal quantum number of n=2 is in an orbital that is larger and higher in energy than an electron with n=1.
The principal quantum number also provides information about the possible subshells and orbitals in an atom. For example, when n=1, there is only one possible subshell, which contains one s orbital. When n=2, there are two possible subshells, one containing one s orbital and one containing three p orbitals. When n=3, there are three possible subshells, one containing one s orbital, one containing three p orbitals, and one containing five d orbitals.
Overall, the principal quantum number plays an important role in understanding the electronic structure and behavior of atoms, and it is a fundamental concept in quantum mechanics.
Electron Orbitals
Electron orbitals are regions of space around the nucleus of an atom where electrons are most likely to be found. The behavior and properties of electrons are described by quantum mechanics, and the concept of orbitals is based on this theory.
Each orbital can hold a maximum of two electrons with opposite spins, and the number of electrons that an orbital can hold depends on the specific type of orbital. There are four types of orbitals: s, p, d, and f. The s orbital is spherical in shape and is the lowest energy orbital, while the p orbital is dumbbell-shaped and has a higher energy level. The d and f orbitals are more complex in shape and have even higher energy levels.
The electrons in an atom are arranged in various energy levels or shells around the nucleus, and each shell contains one or more subshells or orbitals. The shells are labeled using the principal quantum number, n, which can take on integer values from 1 to infinity. The number of subshells in each shell is equal to n, and the number of orbitals in each subshell is determined by the angular momentum quantum number, l