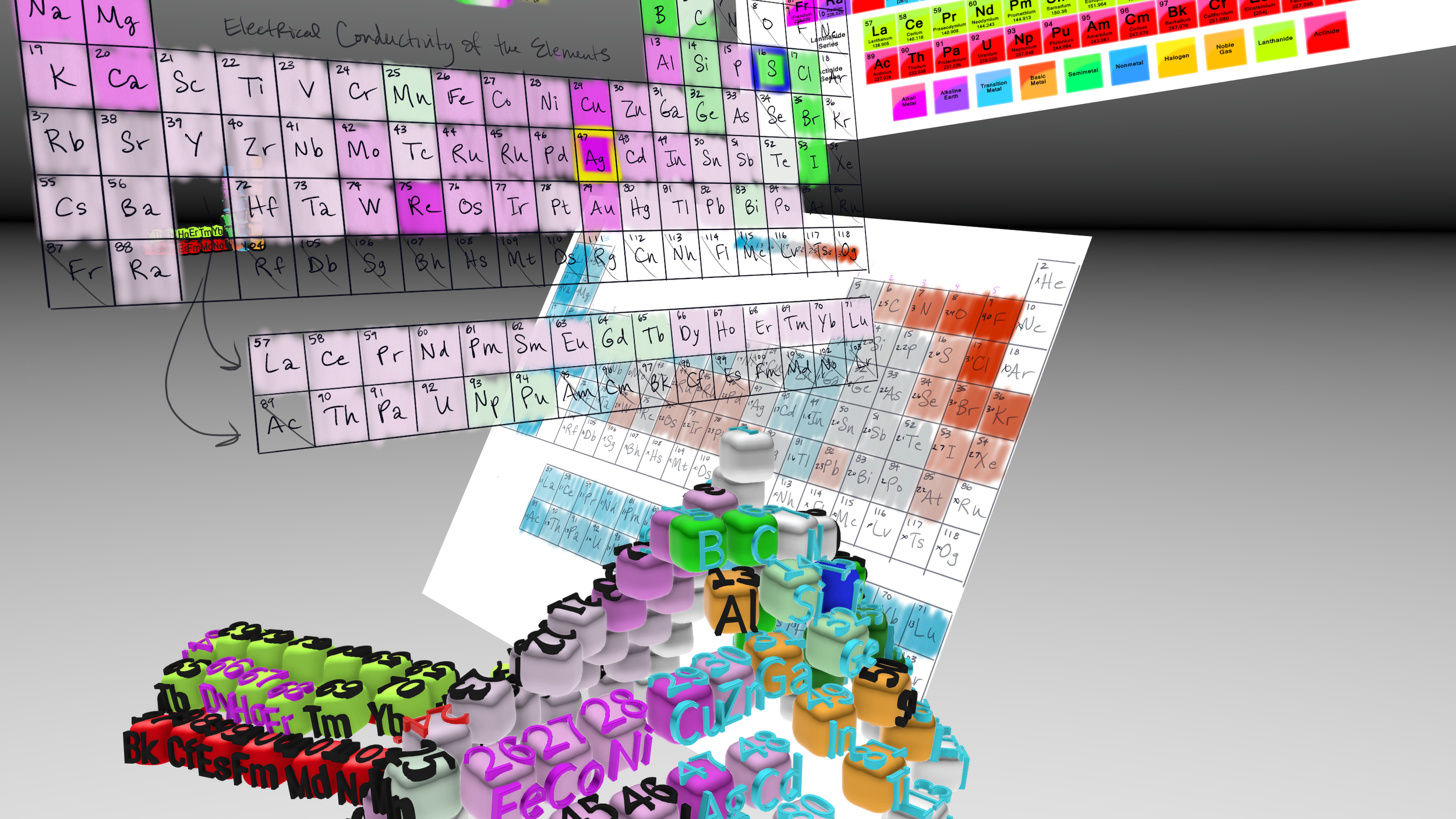
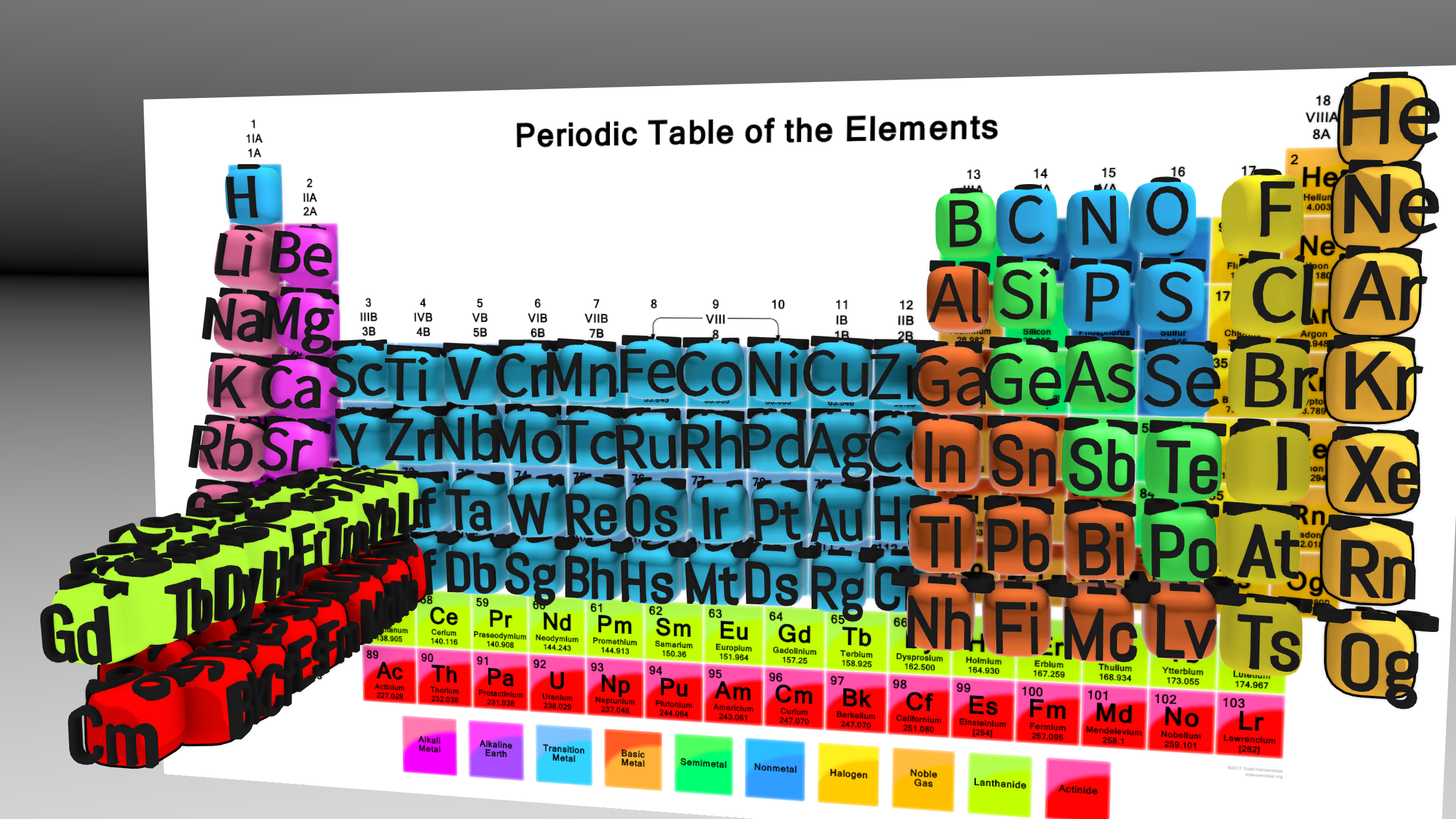
Modern periodic table was last modified in the early 20th century when Henry Moseley discovered that the properties of the elements were related to their atomic number (the number of protons in the nucleus), not their atomic mass. This led to the current configuration which arranges the elements in order of increasing atomic number and groups them by their chemical and physical properties.
New Ways of Looking at the Elements
What is the periodic table of the future?

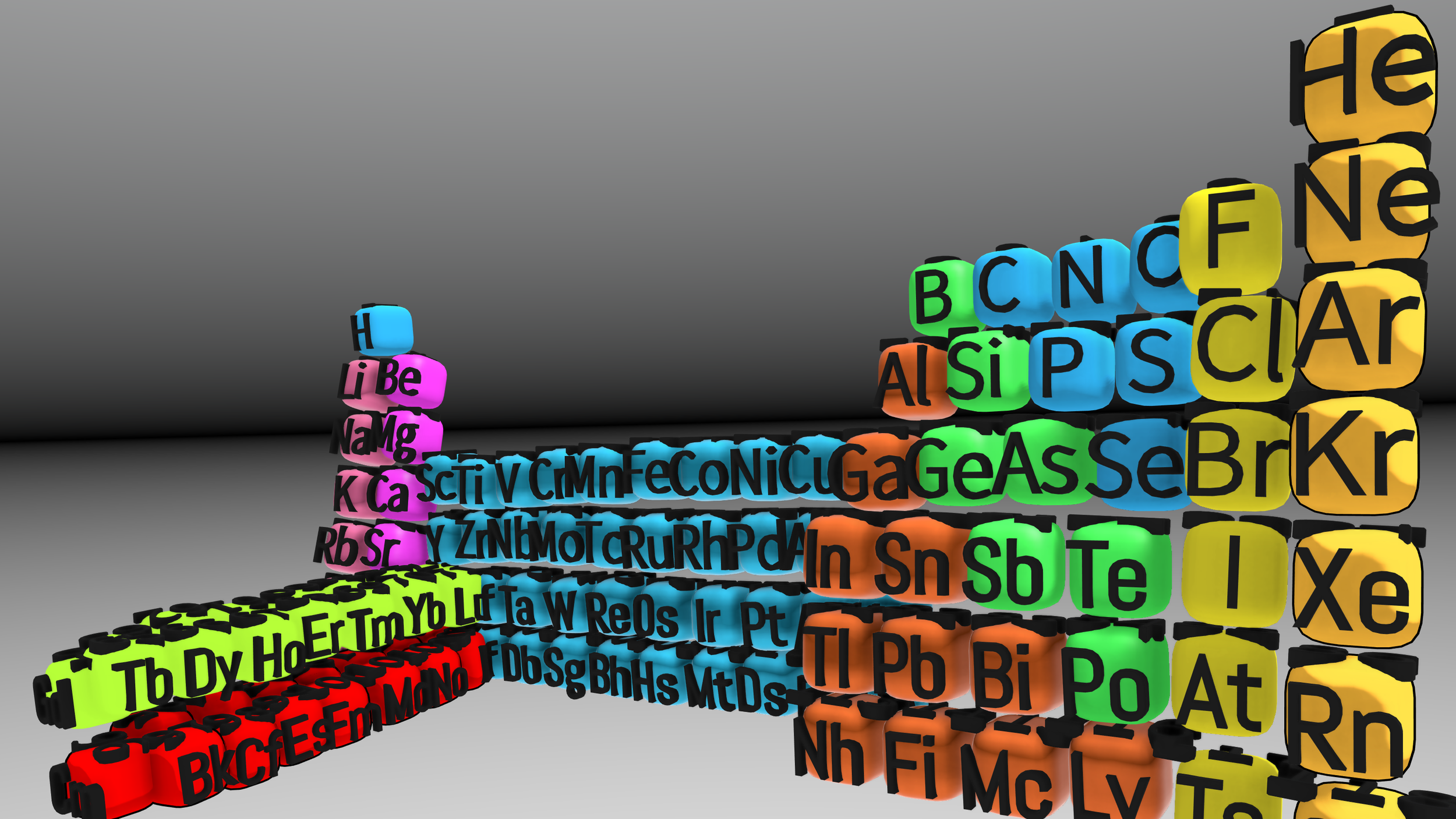
Project Periodic